Gas phase kinetics
Pulsed-Laser-Photolysis (PLP) is used to generate gas phase free
radicals in the presence of reactant molecules. In the case of the CH +
cyclopentadiene (C5H6) reaction the radical is generated by photolysis of bromoform at 266 nm and its concentration measured using Laser Induced Fluorescence
(LIF). A 431-nm laser pulse excites the radicals to its first electronic
excited state and the subsequent radical fluorescence is detected off resonance at 480 nm.
 Kinetic decays are obtained by scanning the delay times between
the pump and the probe lasers.
Under pseudo first order approximation the rate coefficient is
determined by plotting the decay rate as a function of reactant
concentration. The reaction of CH + cyclopentadiene plays a significant role
in carbon growth chemical mechanisms in gas phase environments such as combustion
flames to the interstellar medium.
Kinetic decays are obtained by scanning the delay times between
the pump and the probe lasers.
Under pseudo first order approximation the rate coefficient is
determined by plotting the decay rate as a function of reactant
concentration. The reaction of CH + cyclopentadiene plays a significant role
in carbon growth chemical mechanisms in gas phase environments such as combustion
flames to the interstellar medium.
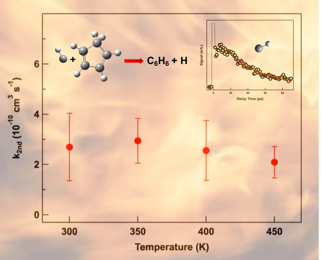
The fast pressure-independent rate coefficients from the CH + cyclopentadiene
reaction advocate the formation of C6H6
isomers that play a crucial role in combustion. The lack of
temperature dependence in the rate coefficients further
suggests that CH cycloaddition, driven by a strong attractive
potential between the reactants, is likely to dominate over
insertion or abstraction mechanisms at room temperature.
Fulvene and benzene are likely to form as major products for
this reaction, supported by thermodynamically accessible
pathways. At combustion relevant temperatures (>800 K),
H-abstraction pathways could become more significant,
becoming a larger source of cyclopentadienyl radicals. Further
kinetic studies at higher temperatures are necessary to confirm
these mechanisms.